Determine the number of bonding electrons and the number of nonbonding electrons in the structure of BeF2. Draw the main Lewis structure of NOF.

Nof Lewis Structure Geometry Hybridization And Polarity Techiescientist
Q no 5.

. I am redo the exam and I have to explain why the choices are correct or wrong. So please help meWhich of the following statement about resonance structures is FALSEA Resonance structure. Draw the main Lewis structure of NOF.
Amazon Luna launches with freebies for Prime subscribers. Drawing the Lewis Structure for NOF. The NOF Lewis structure is very similar to NOCl and NOBr.
Determine the number of bonding electrons and the number of nonbonding electrons in the structure of BeF2. Draw the nonbonding electron using the dot notation and bonding electrons as a bond. Draw nonbonding electrons using the dot notation and bonding electrons as a bond.
Up to 256 cash back Draw the main Lewis structure of NOF. Check the formal charges to be sure that each atom has a formal charge of zero. Mark lone pairs Step 3.
Draw nonbonding electrons using the dot notation and bonding electrons as a bond. You will need to draw the Lewis structure of the NOF molecule to answer this question. Draw sketch Step 2.
Heres how you can draw the ICl 3 lewis structure step by step. Start filling in the gaps now. As nitrogen is the least electronegative element amongst all the three atoms involved it is chosen as the central atom.
2 Posted one year ago. By soetrust March 4 2022 Leave a reply 3. To draw the Lewis structure of NOF we first need to choose a central atom.
What is the Lewis structure of Nitrosyl fluoride NOF. Enter the number of bonding electrons followed by the number of nonbonding electrons separated by a comma in the dot structure. In the NOF Lewis structure.
Drawing the Lewis Structure for NOF In the NOF Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure. HERE THE ANSWERS 320 g CH4 x 1 mole CH4 160 g CH4 200 moles CH4 To get the. ONF Fluorine has 6 dots or electrons oxygen also has 6 and nitrogen has 2.
SOMEONE ASKED What is the final temperature of the methane once the system equilibrates. In this case Nitrogen has 5 valence electrons Oxygen has 6 and Fluorine has 7 and we get 18 when we add them up. 120 120 b What is the hybridization at nitrogen.
Draw the main Lewis structure of NOF. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. The line indicates the bond between S and Cl and each line is two bonding electrons so there are 4 bonding electrons and the remaining are non bonding electrons.
Three possible Lewis structure for NOF are shown below a Calculate formal charges for each atom in the three structure and put the formal charges the atom in the diagrams. Determine the number of bonding electrons and the number of nonbonding electrons in the structure of SCl2. Solution for Draw the main Lewis structure of NOF.
A step-by-step explanation of how to draw the NOF Lewis Dot Structure Nitrosyl fluorideFor the NOF structure use the periodic table to find the total numb. Part B Draw the main Lewis structure of NOF Draw nonbonding electrons using the dot notation and bonding electrons as a bond. The Oxygen and Fluorine atoms are placed on each side of the Nitrogen atom.
_ _ sp 4 _ _ sp 3 o o sp 2 _ _ sp. To determine the lewis structure we take the valence electrons of each atom. In the NOF Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure.
Up to 256 cash back Draw the main Lewis structure of NOF. This preview shows page 4 out of 4 pages. Draw the main lewis structure of nof.
Determine the number of bonding electrons and the number of nonbonding electrons m the structure of BeFeq_2 eq. Amazon Luna special offer for Prime members. Amazon Luna launches with freebies for Prime subscribersAmazon Luna special offer for Prime membersTry Amazon Luna Now.
Determine the number of bonding electrons and the number of nonbonding electrons in the structure of BeF2. What is the Lewis. What is the Lewis dot structure for NOF.
Draw the main Lewis structure of NOF. Drawing the Lewis Structure for NOF In the NOF Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure. Draw the main Lewis structure of NOF.
In NOF the nitrogen is central. In the Lewis structure for NOF there are a total of 18 valence electrons. Draw the main Lewis structure of NOF.
Since Nitrogen is the center of the structure we now have to place Oxygen and Fluorine around the Nitrogen. Try Amazon Luna Now. Draw nonbonding electrons using the dot notation and bonding electrons as a bond.
A What is the predicted bond angle in the NOF molecule. Enter the number of bonding electrons followed by the number of nonbonding electrons separated by a comma in. Drawing Lewis Structure of NOF.
Check the formal charges to be sure that each atom has a formal charge of zero. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Draw the main lewis structure of nof.
The NOF Lewis structure is very similar to NOCl and NOBr. Enter the number of bonding electrons followed by the number of nonbonding electrons separated by a comma in the dot structure.

Draw The Best Lewis Structures Full 3d Structure And State The Geometry For Nof And Secl4 And Include Formal Charges On Atoms With Non Zero Values Study Com
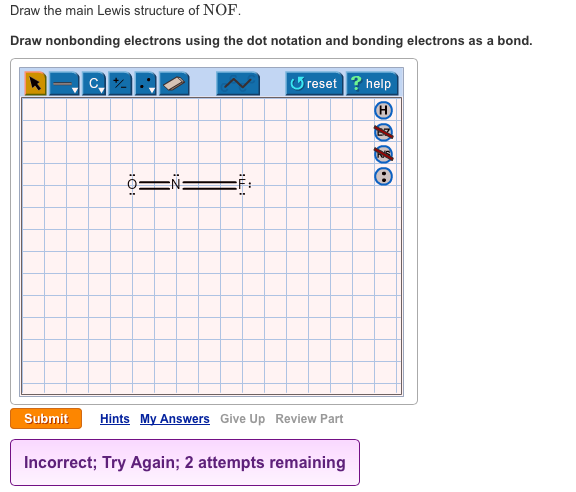
Solved Draw The Main Lewis Structure Of Nof Draw Chegg Com

1 Determine The Number Of Bonding Electrons And The Number Of Nonbonding Electrons In The Structure Of Co 2 2 Draw The Main Lewis Structure Of Nof 3 Determine The Number Of Bonding

Nof Lewis Structure How To Draw The Lewis Structure For Nof Youtube

Draw The Main Lewis Structure Of Nof Draw Nonbonding Electrons Using The Dot Notation And Bonding Brainly Com

Nof Lewis Structure How To Discuss

Nof Lewis Structure How To Draw The Dot Structure For Nof Homeworkavid

Nof Lewis Structure How To Draw The Lewis Structure For Nof Youtube
0 comments
Post a Comment